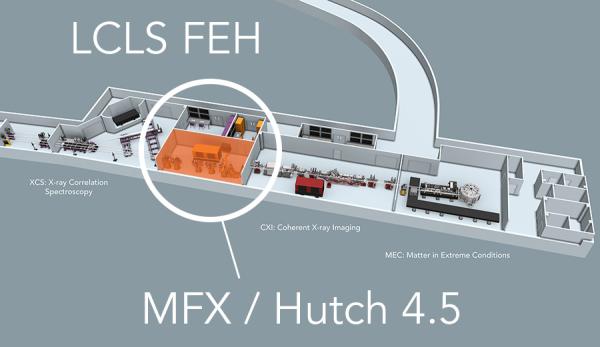
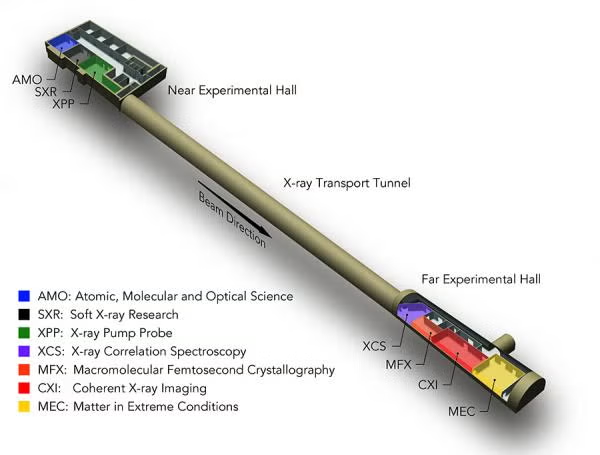
MFX Overview
MFX Full Name
Macromolecular Femtosecond Crystallography
Short Description
The Macromolecular Femtosecond Crystallography (MFX) instrument makes use of the unique brilliant hard X-ray pulses from LCLS to perform a wide variety of experiments utilizing various techniques. The primary capability of MFX is to make use of the high peak power of the focused X-ray beam using the “diffraction-before-destruction” method. This technique prevents damage to a sample during the measurement by performing the measurement faster than the propagation of damage or destruction with ultrashort pulses. This is particularly advantageous for biological samples that suffer from electronic and structural damage during long continuous exposures to X-rays. The MFX instrument builds on the well-developed crystallographic techniques from synchrotrons and from the early days of LCLS to provide an atmospheric pressure, dedicated yet versatile instrument for multi-modal crystallographic measurements at LCLS.
MFX consists of a flexible instrumentation suite to make use of hard X-rays primarily in an atmospheric pressure environment. It enables femtosecond crystallography measurements capable of determining the structure of crystallized biomolecules at room temperature. Other techniques can provide complementary information for a more holistic understanding of molecular processes, such as X-ray Emission Spectroscopy, back-scattering, small and wide angle scattering.
Plans exist for a flexible pump laser system for time-resolved experiments in the femtosecond time scale. MFX will be available for any scientific field requiring use of the LCLS beam, including structural biology, material science, materials in extreme conditions, atomic molecular and optical physics, chemistry and soft condensed matter, provided MFX is deemed most suitable for a particular science case. Samples can be introduced to the X-ray beam either fixed on targets using a high speed and high precision goniometer or using liquid jet systems that can deliver samples to the beam. Beryllium compound refractive lenses will be used for focusing the X-ray beam and control the spot size at the sample. The MFX instrument will operate primarily in the 5-20 keV range with the lower limit set by the atmospheric pressure operation and the upper limit eventually set by the capabilities of LCLS-II.
Scientific Goals
X-ray diffraction has long been used to determine atomic structures of biomolecules. Unfortunately, the same X-rays that are used to probe the structure of the sample also get absorbed and deposit energy in the sample, causing irreparable damage. This often limits the resolution achievable in a particular sample, especially biological samples which are particularly sensitive to damage. X-ray crystallography has been very successful studying biological samples by spreading the damage over as many as billions of molecules in a single crystal, greatly enhancing the diffraction signal. Since the molecules are all identical and precisely aligned in the crystal, the X-ray scattering information is preserved and the structure can be determined. As crystals are reduced in size, they become more sensitive to damage due to the need to irradiate every molecule with more X-rays to get a measurable signal, causing more damage for each molecule.
LCLS offers a way around the damage problem. Since X-ray pulses from LCLS are very intense and very short, it is possible to deliver a higher dose to a sample and record the scattered X-ray information before the damage processes have time to destroy the sample. In other words, an LCLS X-ray pulse can be focused onto a sample, which gets destroyed—but not before the scattered X-rays are already on their way to the detector carrying the information needed to deduce the structure of the molecule. The Macromolecular Femtosecond Crystallography (MFX) instrument offers the possibility of determining structures at resolution beyond the damage limit for samples which are limited by radiation damage at synchrotron sources.
MFX provides a flexible suite of instrumentation for “diffract-before-destroy” studies in structural biology in an atmospheric pressure sample environment. The emphasis of the instrument lies in its multi-modal measurement capabilities, with the X-ray instrumentation being supplemented by additional tools such as nanosecond and femtosecond pump laser systems as well as complementary optical measurement instrumentation.-As a result, MFX is a highly versatile instrument capable of a variety of scattering and spectroscopy techniques, serving several scientific fields such as biology, soft-condensed matter, chemistry and soft condensed matter as well as material science and materials in extreme conditions.
Scientific Programs
Femtosecond Crystallography
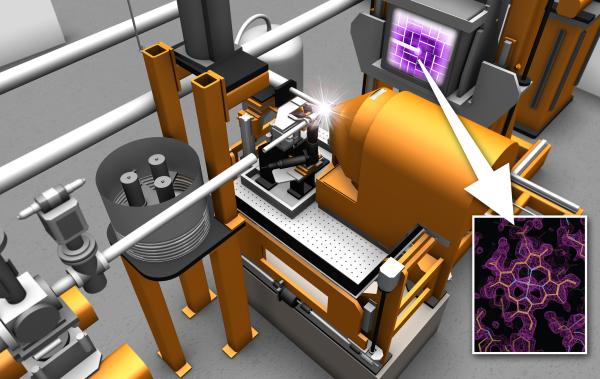
The ultrashort, high intensity pulses of LCLS have the advantage of allowing in principle damage-free higher resolution measurements of radiation-sensitive samples such as metalloenzymes. A series of crystals can be illuminated by LCLS X-ray pulses and the diffraction patterns recorded. A full 3D set similar to conventional protein crystallography can be assembled from many crystals or many shots on the same large crystal and yield the protein structure. The crystals can be delivered into the MFX beam mounted on a goniometer system (Cohen et al, PNAS (2014)) or using a liquid jet (Weierstall et al, Rev. Sci. Instrum. (2010)), LCP jet (Weierstall et al, Nature Communications (2014)), an electrospinning jet (Sierra et al, Acta Cryst D (2012)) or droplets (Su et al, JSR (2021)).
Small and Wide Angle Scattering
Small angle scattering measurements at atmospheric pressure are possible at MFX, with the femtosecond pump laser enabling time-resolved measurements to study ultrafast dynamics in biological samples (Levantino et al, Nature Comm (2015)).
X-ray Emission Spectroscopy
X-ray emission spectroscopy (XES) is enabled by XES spectrometers in the von-Hamos geometry for a range of transition metal K-edges in the hard X-ray regime. The design of the modular XES spectrometer at MFX allows observation of several metals of interest at once, enabling unique insights into the electronic structures of multi-metal active sites commonly found in metallo-proteins.
MFX CONTACT INFO
Leland Gee
MFX Instrument Lead Scientist
(650) 926-3234
lbgee@slac.stanford.edu
Sebastian Dehe
Project Scientist
dehe@slac.stanford.edu
Fred Poitevin
Scientist
(650) 926-5326
fpoitevi@slac.stanford.edu
Daniel Rosenberg
Associate Scientist
(650) 926-4740
djr@slac.stanford.edu
Sandra Mous
Associate Scientist
smous@slac.stanford.edu
Roberto Alonso-Mori
Scientist
(650) 926-4179
robertoa@slac.stanford.edu
Andy Aquila
Scientist
(650) 926-2682
aquila@slac.stanford.edu
Mark Hunter
Scientist
(650) 926-6294
mhunter2@slac.stanford.edu
Ray Sierra
Scientist
(650) 926-3148
rsierra@slac.stanford.edu
Mike Glownia
Laser Scientist
(650) 926-5456
jglownia@slac.stanford.edu
Greg Gate
Laser Scientist
(650) 926-2017
gate@slac.stanford.edu
MFX Hutch
(650) 926-1845
MFX LOCATION